BEAM Up on FDA's Orphan Drug Status for Gene-Editing Therapy
Beam Therapeutics BEAM announced that the FDA has granted an orphan drug designation to its investigational genome-editing candidate, BEAM-101, for the treatment of sickle cell disease (SCD), an inherited blood disorder.
The FDA grants orphan drug designation to support the development of medicines for rare disorders that affect fewer than 200,000 people in the United States. The designation makes the sponsor eligible to receive seven years of market exclusivity following a potential approval, along with the exemption of FDA application fees and tax credits for qualified clinical studies.
Shares of Beam Therapeutics were up 4.3% yesterday following the announcement of the news.
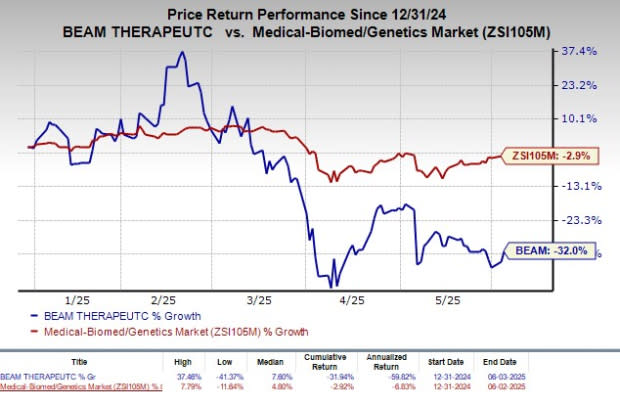
However, the stock has plunged 32% so far this year compared with the industry’s decline of 2.9%.
Image Source: Zacks Investment Research
BEAM’s Ongoing Development Activities With BEAM-101
BEAM-101 is the lead candidate in the company’s hematology franchise. The phase I/II BEACON study is currently evaluating BEAM-101 for the treatment of adult patients with SCD.
In December 2024, the company announced new safety and efficacy data from the BEACON study evaluating BEAM-101 in SCD patients with severe vaso-occlusive crises.
The data showed that treatment with BEAM-101 led to a robust and durable increase in fetal hemoglobin and a reduction in sickle hemoglobin.
The data suggested that the initial safety profile of BEAM-101 was consistent with busulfan conditioning and autologous hematopoietic stem cell transplantation.
Updated data from the BEACON study is expected to be presented at the European Hematology Association conference shortly.
BEAM's Competition in the Target Market
Besides Beam Therapeutics, a few other companies are also utilizing CRISPR/Cas9 nuclease technology, including CRISPR Therapeutics CRSP and Intellia Therapeutics NTLA.
CRISPR Therapeutics is rapidly leveraging its CRISPR/Cas9 gene-editing platform to develop therapies for the treatment of hemoglobinopathies, cancer and other diseases.
CRISPR Therapeutics and partner Vertex Pharmaceuticals’ VRTX CRISPR/Cas9 gene-edited therapy, Casgevy, was approved for two blood disorder indications, SCD and transfusion-dependent beta thalassemia, in several countries in 2023/2024.
VRTX leads global development, manufacturing, and commercialization of Casgevy and splits program costs and profits worldwide in a 60:40 ratio with CRSP.
Intellia Therapeutics is a clinical-stage gene editing company focused on developing drugs with CRISPR-based therapies.
Intellia is developing nexiguran ziclumeran (nex-z, also known as NTLA-2001), an investigational in vivo CRISPR-based therapy for treating transthyretin (ATTR) amyloidosis. NTLA has collaborated with Regeneron for nex-z.
Intellia is also developing its wholly-owned NTLA-2002, an investigational in vivo CRISPR-based therapy for the treatment of hereditary angioedema.
Both nex-z and nex-z are in late-stage development.
BEAM's Zacks Rank
Beam Therapeutics currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Vertex Pharmaceuticals Incorporated (VRTX) : Free Stock Analysis Report
Beam Therapeutics Inc. (BEAM) : Free Stock Analysis Report
Intellia Therapeutics, Inc. (NTLA) : Free Stock Analysis Report
CRISPR Therapeutics AG (CRSP) : Free Stock Analysis Report
This article originally published on Zacks Investment Research (zacks.com).
Zacks Investment Research
Disclaimer: Investing carries risk. This is not financial advice. The above content should not be regarded as an offer, recommendation, or solicitation on acquiring or disposing of any financial products, any associated discussions, comments, or posts by author or other users should not be considered as such either. It is solely for general information purpose only, which does not consider your own investment objectives, financial situations or needs. TTM assumes no responsibility or warranty for the accuracy and completeness of the information, investors should do their own research and may seek professional advice before investing.
Most Discussed
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10